CH3CHO Lewis Structure Resonance
Introduction:
In the fascinating world of chemistry, Lewis structures play a crucial role in understanding molecular bonding and geometry. They provide a simple and visual representation of molecules and their electron arrangements. Among the numerous molecules, one that stands out is acetaldehyde (CH3CHO). This compound not only serves as a valuable industrial chemical but also acts as a key intermediate in organic synthesis.
Understanding Lewis Structures:
Lewis structures, named after the renowned chemist Gilbert N. Lewis, are diagrams that illustrate the arrangement of atoms and lone pairs in a molecule. By using Lewis structures, chemists can predict the reactivity, geometry, and other important properties of compounds. Drawing Lewis structures involves identifying the central atom, placing surrounding atoms, and adding lone pairs as needed.
The Structure of CH3CHO:
CH3CHO, also known as acetaldehyde or ethanal, consists of two carbon atoms, three hydrogen atoms, and one oxygen atom. The carbon atom with three hydrogens forms the “methyl” group, while the other carbon atom binds to the oxygen atom, forming the “aldehyde” functional group. Identifying the central atom is essential, and in this case, the carbon atom in the aldehyde group takes that position.
Introduction to Resonance:
Resonance is a fascinating concept in chemical bonding where a molecule can have multiple Lewis structures without changing its actual structure. These various representations are called “resonance structures.” The true structure of the molecule is considered a hybrid or blend of all the resonance structures, and it is more stable than any individual representation.
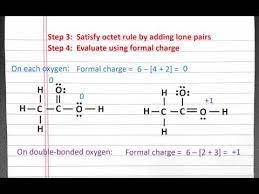
Resonance in CH3CHO Lewis Structure:
When examining the Lewis structure of CH3CHO, we encounter an intriguing phenomenon: resonance. The oxygen atom can form a double bond with the carbon atom in the aldehyde group or a single bond with the adjacent carbon atom. This duality gives rise to multiple resonance structures for CH3CHO. The first resonance structure places a double bond between the carbon and oxygen atoms, while the second resonance structure features a double bond between the two carbon atoms. The actual structure is a hybrid of these two forms.
Properties and Characteristics of CH3CHO:
- Acetaldehyde possesses unique physical and chemical properties.
- It is a colorless, volatile liquid with a pungent odor.
- It is highly flammable and is soluble in water.
- In terms of reactivity, CH3CHO is an important precursor in the synthesis of various chemicals and materials.
- Its Lewis structure and resonance are significant factors in understanding its behavior in different reactions.
Applications and Importance:
The applications of CH3CHO extend to various industries. It serves as a vital building block for the production of pharmaceuticals, plastics, and perfumes. Moreover, acetaldehyde plays a significant role in the creation of resins, fuel additives, and rubber products. Understanding its Lewis structure and resonance is crucial for predicting and controlling its involvement in these processes.
Effect of Resonance on Reactivity:
The resonance hybrid of CH3CHO contributes to its stability, making it less reactive compared to a molecule without resonance. The presence of resonance-stabilized intermediates in reaction pathways can impact the overall reactivity of the compound. This characteristic of CH3CHO is vital in certain synthetic routes, where controlling reactivity is essential for obtaining desired products.
Comparing Lewis Structures Without Resonance:
To grasp the impact of resonance on the stability and reactivity of CH3CHO, let’s compare it to a molecule without resonance. Consider a hypothetical compound, XYZ, which has a similar molecular formula but lacks the capability for resonance stabilization. By contrasting the properties of XYZ with those of CH3CHO, we can better appreciate the significance of resonance in the latter.
Other Examples of Resonance in Organic Molecules:
Resonance is not exclusive to CH3CHO; numerous organic molecules exhibit this intriguing phenomenon. For instance, benzene, a fundamental aromatic compound, showcases resonance structures with delocalized electrons. Exploring other examples of resonance in different organic molecules sheds light on its widespread occurrence and its effects on molecular behavior.
Experimental Methods for Analyzing Resonance:
While Lewis structures and resonance are essential theoretical concepts, experimental methods provide valuable insights into these phenomena. Techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy enable researchers to study molecular structures and interactions. Additionally, X-ray crystallography offers detailed information on molecular arrangements in crystals, providing complementary data on resonance in certain compounds.
Limitations of Lewis Structures and Resonance:
As powerful as Lewis structures and resonance are, they do have limitations. In some cases, they may not provide an accurate representation of a molecule’s true behavior. Particularly in molecules with extensive delocalization or radical species, the conventional Lewis structure model falls short. Recognizing these limitations encourages chemists to explore other theoretical models and experimental data.
Real-life Applications:
The concept of resonance extends beyond the confines of the laboratory and finds its way into everyday life. Understanding resonance helps in the development of pharmaceuticals, agrochemicals, and advanced materials. It also plays a role in designing efficient energy storage systems and catalysts for industrial processes. Emphasizing real-life applications of resonance bridges the gap between scientific concepts and their tangible impact on society.
Conclusion:
In conclusion, the study of CH3CHO Lewis structure resonance sheds light on the intricacies of chemical bonding and reactivity. Resonance provides a deeper understanding of the stability and behavior of this essential compound. By recognizing the significance of resonance in acetaldehyde and other organic molecules, chemists can harness this knowledge to drive innovation and improve various industrial processes.
FAQs:
- Is acetaldehyde harmful to human health?
- Acetaldehyde can be harmful if exposure occurs at high levels. It is considered a potential carcinogen and can cause respiratory and skin irritation. Proper safety precautions should be followed when handling this compound.
- Can resonance occur in inorganic compounds?
- Resonance is a phenomenon primarily observed in organic compounds, where π electrons are delocalized. Inorganic compounds, which often involve d-orbitals, do not commonly exhibit resonance structures.
- How is CH3CHO used in the perfume industry?
- Acetaldehyde’s pleasant fruity odor makes it a valuable ingredient in the perfume industry. It serves as a key component in various fragrance formulations.
- What are some alternatives to acetaldehyde in organic synthesis?
- In some cases, formaldehyde (HCHO) or other aldehydes can serve as alternatives to acetaldehyde in organic synthesis.
- Can resonance be observed experimentally?
- Resonance itself cannot be directly observed experimentally. However, its effects on molecular properties and reactivity can be detected through various experimental techniques, such as spectroscopy.